ALKINDI SPRINKLE is designed for accurate dosing
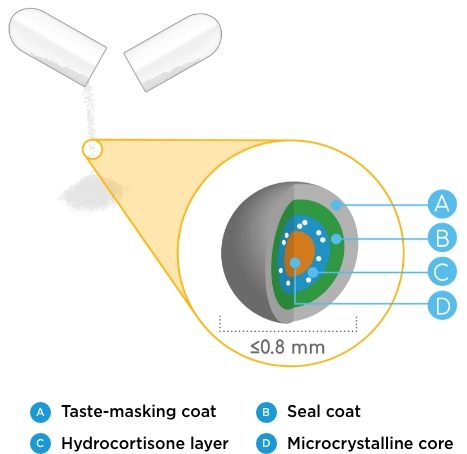
ALKINDI SPRINKLE low-dose hydrocortisone granules are1-3:
- Finished with a kid-friendly coating to help mask the taste when taken as directed and may be preferred over crushed tablets
- Sized below the FDA limit for choking (≤0.8 mm)
- Uniform in size and surface
- Available in 4 flexible dosage strengths: 0.5 mg, 1 mg, 2 mg, and 5 mg
Review the ALKINDI SPRINKLE Dosage and Administration Guide

Avoid under- or overdosing in adrenal insufficiency treatment
Hydrocortisone has a narrow therapeutic dosing window.2 Treating children outside this window can result in poor health outcomes beyond youth and into adulthood.4-7
LONG-TERM CONSEQUENCES OF EXCESS CORTISOL
- Short stature4
- Cardiovascular disease5
- Excessive weight gain4
- Hypertension4-6
- Cushing’s syndrome7
- Insulin resistance5,7
- Osteoporosis7
LONG-TERM CONSEQUENCES OF DEFICIENT CORTISOL
- Adrenal crisis4
- Malaise/illness4
- Hypotension4-5
- Weight loss4,7
- Salt cravings4
- Electrolyte disturbances7
- Virilization and rapid growth in congenital adrenal hyperplasia (CAH)4,7

Prescribing and treating children with adrenal insufficiency using an accurate dosing regimen is vital now and later
- High doses of hydrocortisone (>25 mg/m2/day) early in the disease course in children with adrenal insufficiency can lead to decreased growth resulting in shorter adult height8
- Children and adolescents with CAH have a higher risk of obesity than those with normal health, partially due to the glucocorticoid dosage9
- Adult patients with CAH are at increased risk for obesity, hypertension, osteoporosis, and reduced quality of life10
No effect is claimed for ALKINDI SPRINKLE on these conditions.
Eton Cares is here to help
Get your pediatric patients accurate treatment for adrenal insufficiency with the help of Eton Cares.
Get Help From Eton CaresALKINDI SPRINKLE demonstrated long-term safety and efficacy
ALKINDI SPRINKLE study designs
The safety and efficacy of ALKINDI SPRINKLE for the treatment of patients with primary adrenal insufficiency were established in a phase 3, open-label, single-dose study on the basis of measuring serum cortisol concentrations and observing any adverse events after administration of ALKINDI SPRINKLE.11,12
Three Cohorts of Patients (N=24) With Primary Adrenal Insufficiency11,12
Cohort 1
≥2 to <6 years old
(n=12)
Cohort 2
≥28 days to <2 years
(n=6)
Cohort 3
Birth to <28 days old
(n=6)
The primary endpoint was the maximum levels of serum cortisol concentration up to 240 minutes after taking ALKINDI SPRINKLE using blood samples taken at 0, 60, and 240 minutes post dose.
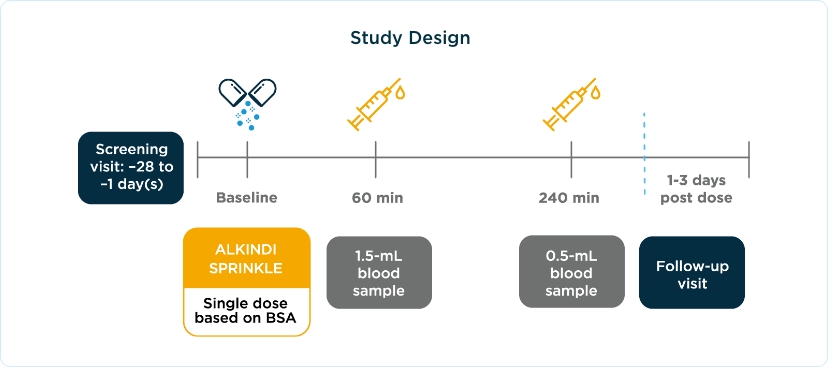

BSA, body surface area.
Accurate dosing helps your pediatric patients keep their eyes on the future

ALKINDI SPRINKLE achieved clinically relevant increases in serum cortisol.11,12
- Patients in cohorts 1 and 2 achieved a similar rise in cortisol
- A higher peak cortisol concentration was seen in patients in cohort 3 due to higher dosing (mg/m2 basis)
- Median serum cortisol was 19.40 μg/dL at 60 minutes after ALKINDI SPRINKLE
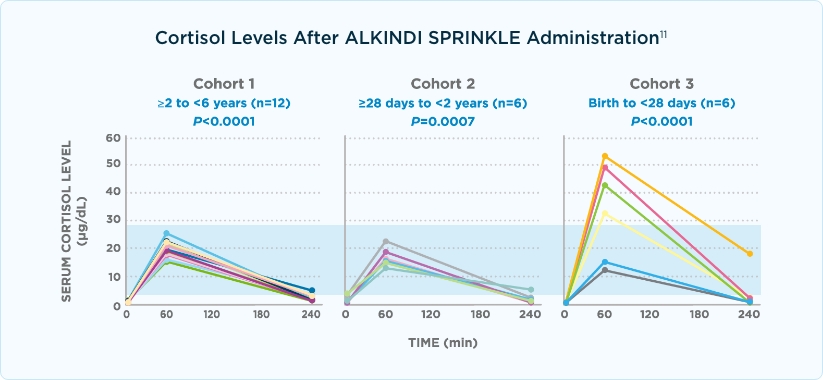

Graph shading represents the normal peak cortisol ranges for healthy children.11,13,14
Absolute difference between Cmax (at 60 minutes) and baseline geometric mean serum cortisol concentrations: cohort 1, P<.0005; cohort 2, P=.0313; and cohort 3, P=.0026.
ALKINDI SPRINKLE demonstrated long-term safety and efficacy*
Accurate dosing demonstrated therapeutic cortisol concentrations and normal growth-rate patterns over 2.5 years in a safety study.12,15,*
- 18 of 24 children in the phase 3 study continued in a 2.5-year long-term safety study
- Primary endpoint was the nature and occurrence of adverse events observed throughout the study
- Patients received ALKINDI SPRINKLE 3 times daily until they met the study withdrawal criteria (ie, adrenal crisis, ALKINDI SPRINKLE commercially available, or study was discontinued)
17 patients† experienced growth-rate patterns in the ranges of healthy children
No adverse progression of sexual development was reported by all patients (17) with CAH
No significant accelerated or reduced growth was reported, and no trends were seen that might indicate over- or undertreatment
All 12 patients who completed the study achieved appropriate serum cortisol concentrations and did not experience adrenal crisis during the study period
†All 17 participants with CAH experienced normal growth rate patterns; the exception was 1 patient with hypopituitarism.
ALKINDI SPRINKLE provided consistent serum cortisol concentrations*
Study A
ALKINDI SPRINKLE 10 mg has a similar pharmacokinetic profile to hydrocortisone 10-mg tablet12,16
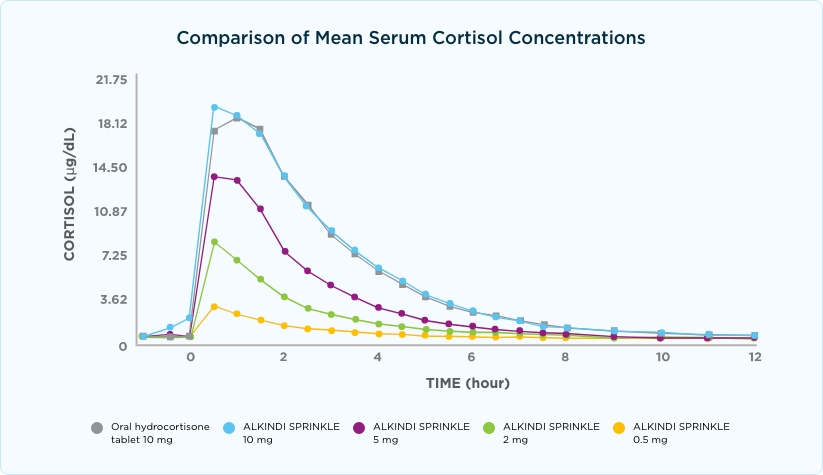

Consistent cortisol concentrations for patients with adrenal insufficiency can help improve quality of life17,18
- Nonphysiologic glucocorticoid replacement therapy may reduce quality of life18
- Frequent hypocortisolemia may lead to low energy, decreased mental concentration, and daytime somnolence17
Review articles did not include ALKINDI SPRINKLE. Therefore, no representations are made for the effect of ALKINDI SPRINKLE on the conditions described.
Study B
ALKINDI SPRINKLE 20 mg was bioequivalent to hydrocortisone 20-mg tablet and was 87% bioavailable compared with hydrocortisone IV. No treatment-emergent adverse events were associated with ALKINDI SPRINKLE.12
See below for Important Safety Information, including Adverse Reactions.
Study C
ALKINDI SPRINKLE 20 mg had similar bioequivalence and bioavailability vs Cortef® (hydrocortisone) 20-mg tablets in fasted and fed states. No serious adverse events, treatment-emergent adverse events (TEAEs) that led to withdrawal from the study, or TEAEs with a suspected causal relationship to any study medication were reported.12
*In dexamethasone-supressed healthy adult male and female patients (Study A: N=16; Study B: N=14; Study C: N=51).
ALKINDI SPRINKLE was generally well tolerated
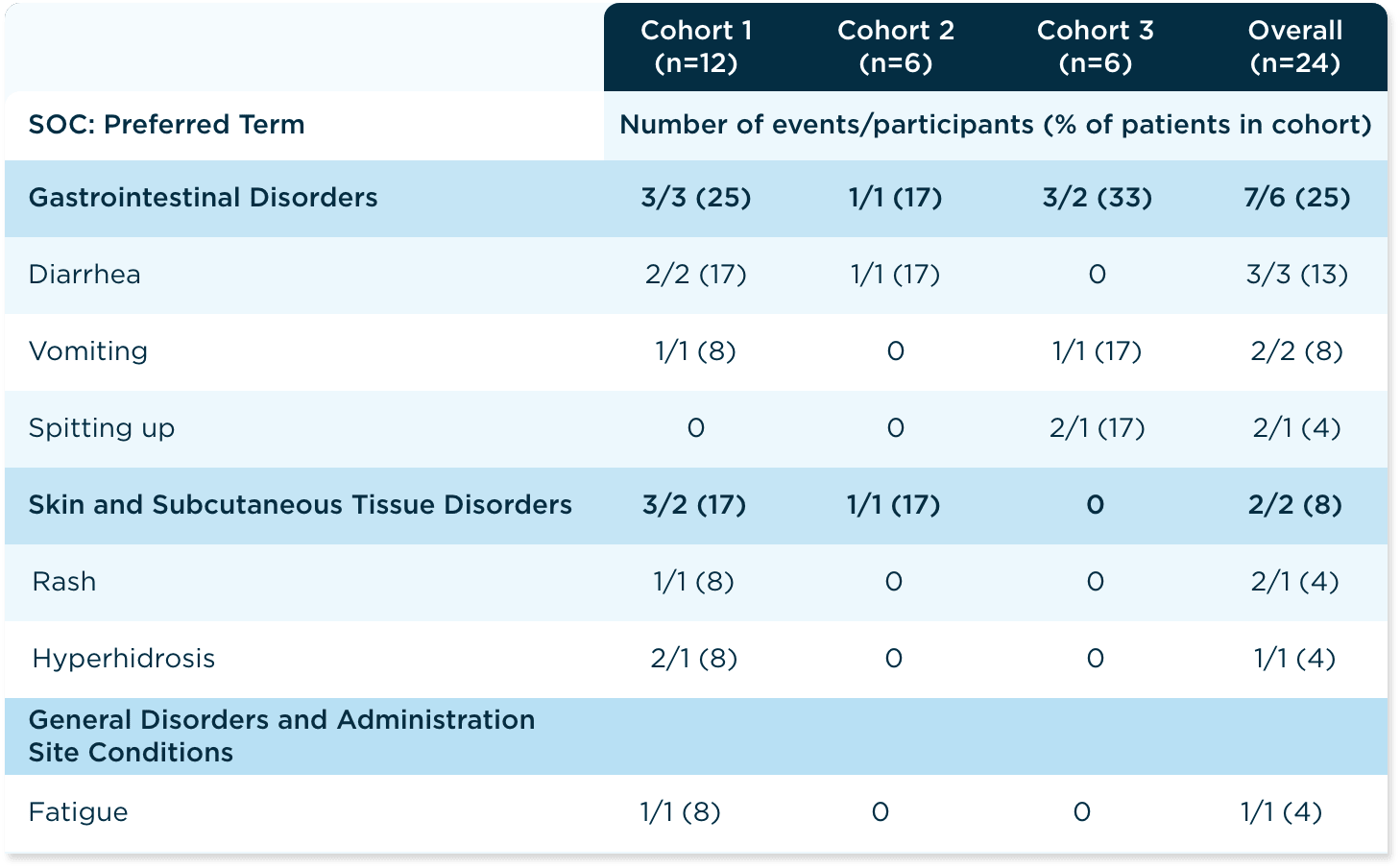
The safety profile of ALKINDI SPRINKLE low-dose hydrocortisone oral granules was studied in the phase 3, open-label, single-dose replacement study for primary adrenal insufficiency, including CAH12
The safety profile of ALKINDI SPRINKLE noted in this phase 3 study was similar to that observed in the bioequivalence studies with healthy adult patients and in the 2.5-year long-term study with children with primary adrenal insufficiency.12,15
Summary of TEAEs by SOC and Preferred Terms

ALKINDI SPRINKLE helps provide accurate, individualized dosing for children with adrenal insufficiency
Learn more about accurate hydrocortisone treatment for children.
References: 1. ALKINDI SPRINKLE. Package insert. Eton Pharmaceuticals, Inc; 2024. 2. Neumann U, Whitaker MJ, Wiegand S, et al. Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clin Endocrinol (Oxf). 2018;88(1):21-29. doi:10.1111/cen.13447 3. Center for Drug Evaluation and Research. Guidance for industry: size of beads in drug products labeled for sprinkle. Published May 2012. Accessed May 5, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/size-beads-drug-products-labeled-sprinkle-rev1 4. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. doi:10.1210/jc.2015-1710 5. Han TS, Conway GS, Willis DS, et al. Relationship between final height and health outcomes in adults with congenital adrenal hyperplasia: United Kingdom congenital adrenal hyperplasia adult study executive (CaHASE). J Clin Endocrinol Metab. 2014;99:E1547-E1555. doi:10.1210/jc.2014-1486 6. Oprea A, Bonnet NCG, Pollé O, Lysy PA. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther Adv Endocrinol Metab. 2019;10:2042018818821294. doi:10.1177/2042018818821294 7. Debono M, Newell Price J, Ross RJ. Novel strategies for hydrocortisone replacement. Best Pract Res Clin Endocrinol Metab. 2009;23(2):221-232. doi:10.1016/j.beem.2008.09.010 8. Hauffa BP, Winter A, Stolecke H. Treatment and disease effects on short-term growth and adult height in children and adolescents with 21-hydoxylase deficiency. Klin Padiatr. 1997;209(2);71-77. doi:10.1055/s-2008-1043931 9. Volkl TMK, Simm D, Beier C, et al. Obesity among children and adolescents with classic adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 2006;117(1):e98-e105. doi:10.1542/peds.2005-1005 10. Han TS, Walker R, Arlt W, et al. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nature Rev Endocrinol. 2014;10(2):115-124. doi:10.1038/nrendo.2013.239 11. Neumann U, Whitaker MJ, Wiegand S, et al. Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clin Endocrinol (Oxf). 2018;88(1):21-29. doi:10.1111/cen.13447 12. Data on file. Eton Pharmaceuticals, Inc. Deer Park, IL. 13. Volovitz B, Kauschansky A, Nussinovitch M, Harel L, Varsano I. Normal diurnalvariation in serum cortisol concentration in asthmatic children treated with inhaled budesonide. J Allergy Clin Immunol. 1995;96(6 pt 1):874-878. doi:10.1016/s0091-6749(95)70222-9 14. Debono M, Ghobadi C, Rostami- Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94(5):1548-1554. doi:10.1210/jc.2008-2380 15. Neumann U, Braune K, Whitaker MJ, et al. A prospective study of children aged 0-8 years with CAH and adrenal insufficiency treated with hydrocortisone granules. J Clin Endocrinol Metab. 2021;106(3):e1433-e1440. doi: 10.1210/clinem/dgaa626 16. Whitaker MJ, Spielmann S, Digweed D, et al. Development and testing in healthy adults of oral hydrocortisone granules with taste masking for the treatment of neonates and infants with adrenal insufficiency. J Clin Endocrinol Metab. 2015;100(4):1681-1688. doi:10.1210/jc.2014-4060 17. Porter J, Blair J, Ross RJ. Is physiological glucocorticoid replacement important in children? Arch Dis Child. 2017;102:199-205. doi:10.1136/archdischild-2015-309538 18. Guran T. Latest insights on the etiology and management of primary adrenal insufficiency in children. J Clin Res Pediatr Endocrinol. 2017;9(suppl 2):9-22. doi:10.4274/jcrpe.2017.S002
