
ALKINDI SPRINKLE is a treatment of pediatric-specific hydrocortisone oral granules that manages cortisol levels to help facilitate accurate and individualized dosing in infants, children, and adolescents (neonate to <17 years old) with adrenal insufficiency, including congentical adrenal hyperplasia (CAH).1,2*
*The safety and effectiveness of ALKINDI SPRINKLE have not been established in patients over 17 years of age.1Avoid under- or overdosing in the treatment of adrenal insufficiency
Hydrocortisone has a narrow therapeutic dosing window.2 Treating children outside this window can result in poor health outcomes beyond youth and into adulthood.3-6
Dosing inaccuracy is possible with pill manipulation and compounded formulations at home and at the compounding pharmacy
There is a risk of under- or overdosing due to imprecise measurements in crushing or splitting tablets and the variability in physical dimensions of the resulting product.7

Guidelines exist for healthcare professionals; however, the methods caregivers use to manipulate tablets may vary depending on7:
- Required dose
- Type of product(s)
- HCP advice
- Home equipment
Compounding at the pharmacy
Issues with compounded hydrocortisone8:
21.4% of batches lacked uniformity of net mass or drug content*
3.6% completely failed to contain any trace of the labeled drug
*Evaluation of 56 batches containing 1125 capsules.
Accurate hydrocortisone dosing for your patients is possible with ALKINDI SPRINKLE*
ALKINDI SPRINKLE is available in 4 strengths within color-coded capsules for flexible, accurate dosing.
ALKINDI SPRINKLE can help provide accurate dosing tailored to the cortisol needs of your pediatric patients, with the lowest, most effective dose.
*When the entire dose is administered when and as directed.
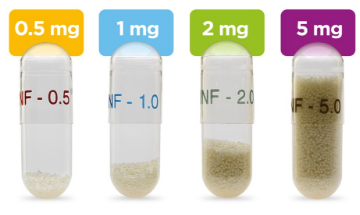
Capsules shown are not actual size.
No pill splitting
No pill crushing
No compounding
Just sprinkles
See how ALKINDI SPRINKLE helps your pediatric patients keep their eyes on the future
Prescribing ALKINDI SPRINKLE
All prescriptions for ALKINDI SPRINKLE need to be sent directly to Anovo® Specialty Pharmacy using either of the methods below. Once ALKINDI SPRINKLE has been prescribed, your patients will have access to Eton Cares,* a comprehensive patient support program.
Via Fax
- Complete the ALKINDI SPRINKLE Patient Referral Form, including prescription details and important information for the benefits investigation process
- Send via fax (1-855-813-2039) to Anovo Specialty Pharmacy
Via e-Script
- Initiate e-Script from EMR system
- Select “Anovo #5” for pharmacy
Anovo Specialty Pharmacy will follow up with a call to your office to obtain the patient information required for the benefits investigation process.
*Restrictions, limitations, and/or eligibility requirements apply.
Stay informed and up to date with ALKINDI SPRINKLE
Sign up now to receive the latest news and information for your practice, patients, and their caregivers.
References: 1. ALKINDI SPRINKLE. Package insert. Eton Pharmaceuticals, Inc; 2024. 2. Neumann U, Whitaker MJ, Wiegand S, et al. Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clin Endocrinol (Oxf). 2018;88(1):21-29. doi:10.1111/cen.13447 3. Han TS, Conway GS, Willis DS, et al. Relationship between final height and health outcomes in adults with congenital adrenal hyperplasia: United Kingdom congenital adrenal hyperplasia adult study executive (CaHASE). J Clin Endocrinol Metab. 2014;99:E1547‐E1555. doi:10.1210/jc.2014-1486 4. Oprea A, Bonnet NCG, Pollé O, Lysy PA. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther Adv Endocrinol Metab. 2019;10:2042018818821294. doi:10.1177/2042018818821294 5. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. doi:10.1210/jc.2015-1710 6. Debono M, Newell Price J, Ross RJ. Novel strategies for hydrocortisone replacement. Best Pract Res Clin Endocrinol Metab. 2009;23(2):221-232. doi:10.1016/j.beem.2008.09.010 7. Watson C, Webb EA, Kerr S, Davies JH, Stirling H, Batchelor H. How close is the dose? Manipulation of 10 mg hydrocortisone tablets to provide appropriate doses to children. Int J Pharm. 2018;545(1-2):57-63. doi:10.1016/j.ijpharm.2018.04.054 8. Neumann U, Burau D, Spielmann S, et al. Quality of compounded hydrocortisone capsules used in the treatment of children. Eur J Endocrinol. 2017;177(2):239-242. doi:10.1530/EJE-17-0248
