
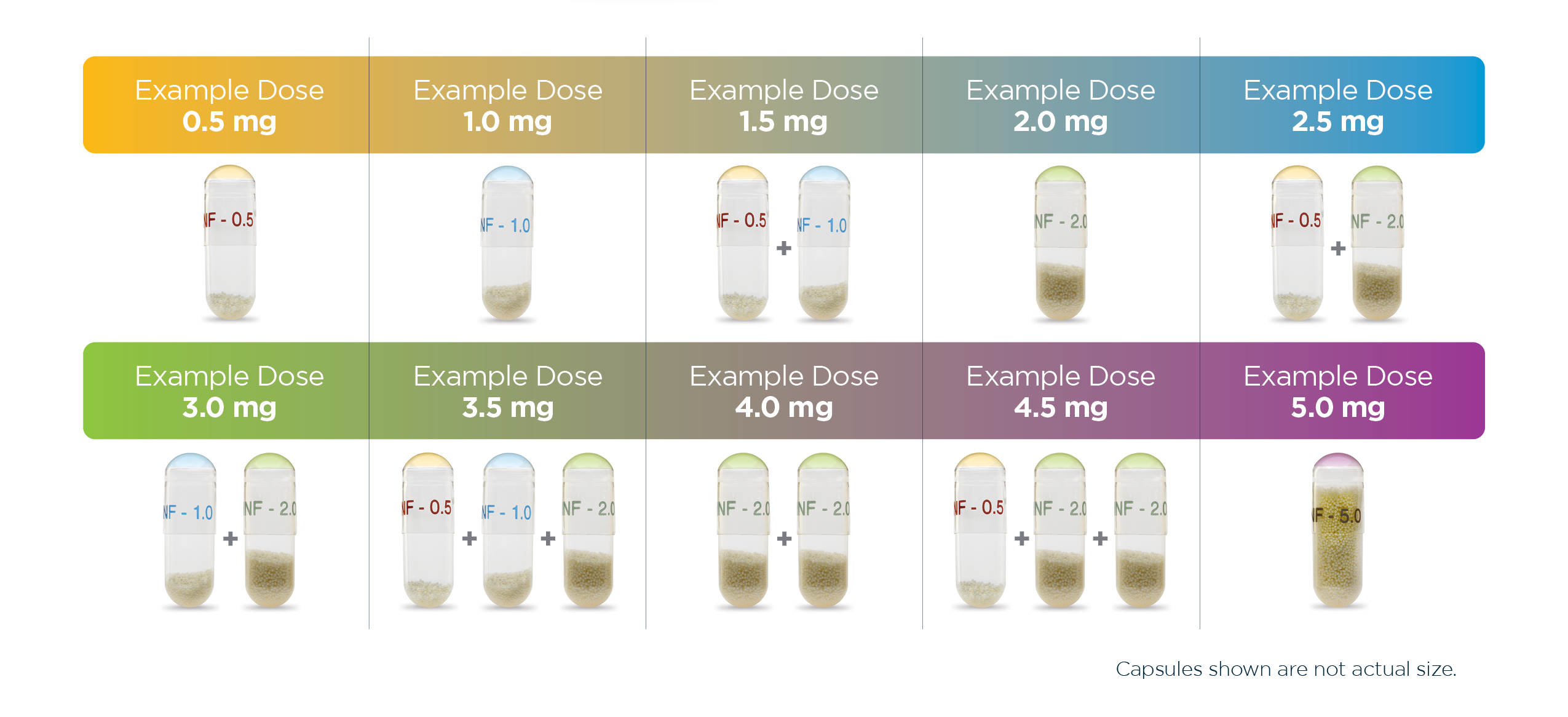
Recommended dosing of ALKINDI SPRINKLE for replacement cortisol treatment1,2
ALKINDI SPRINKLE® (hydrocortisone) oral granules 5 mg sprinkled on soft food and administered was found to be bioequivalent to dry granules administered directly into the mouth.3
For healthy dexamethasone-suppressed adult male patients who received a single 5-mg dose in a 3-period, crossover study, Cmax, AUC0-t, and AUC0-inf did not differ when ALKINDI SPRINKLE was administered with soft food, such as yogurt, or directly into the mouth.
Cmax, maximum concentration; AUC0-t, area-under-the-concentration curve from time zero to time t; AUC0-inf, area-under-the-concentration curve from time zero to infinity.

Dosing at home
Caregivers* who prepared doses of hydrocortisone from manipulation of 10-mg tablets indicated that >50% of doses could not be prepared from quartering tablets.4
- For children <6 years old, 191 doses were reported, ranging from 0.25 to 15 mg
- 43.2% of those doses were divisible by 2.5 mg
*N=159 survey respondents.
No pill splitting
No pill crushing
No compounding
Just sprinkles
Administering ALKINDI SPRINKLE
-

Check expiration and prepare
Check the expiration date on the ALKINDI SPRINKLE bottle. Do not use after the expiration date listed on the bottle.
Then, remove the prescribed dose of ALKINDI SPRINKLE capsules from the bottle.
-
Hold and tap
Hold capsule with the writing at the top. Tap the capsule to make sure the granules fall to the bottom.
-
Squeeze
Gently squeeze the bottom of the capsule to loosen the top from the bottom.
-
Twist and remove
Carefully twist off the top of the capsule.
-
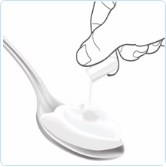
Sprinkle
ALKINDI SPRINKLE can be given by spoon with soft food or sprinkled directly into the child’s mouth.
-
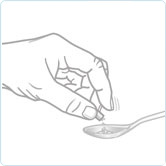
Tap
Tap the capsule to ensure all the granules are removed. Avoid wetting the capsule on the tongue or soft food as this may result in granules remaining in the capsule.
-
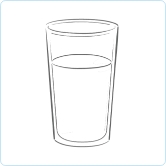
Give fluids
After giving ALKINDI SPRINKLE, give the child a sip of fluids, such as water, milk, breast milk, or formula, right away to ensure all granules are swallowed.
ALKINDI SPRINKLE granules may sometimes be seen in stools because the center of the granule is not absorbed in the gut after the drug has been released.
ALKINDI SPRINKLE has a texture that may be new to most patients
Some pediatric patients may object to the texture of ALKINDI SPRINKLE in their mouth. It’s best to give with soft food. Watch a short video of a family administering ALKINDI SPRINKLE.
Ready to prescribe ALKINDI SPRINKLE?
Eton Cares and Anovo® Specialty Pharmacy are here to help.
References: 1. Oprea A, Bonnet NCG, Pollé O, Lysy PA. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther Adv Endocrinol Metab. 2019;10:2042018818821294. doi:10.1177/2042018818821294 2. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. doi:10.1210/jc.2015-1710 3. Data on file. Eton Pharmaceuticals, Inc. Deer Park, IL. 4. Watson C, Webb EA, Kerr S, Davies JH, Stirling H, Batchelor H. How close is the dose? Manipulation of 10 mg hydrocortisone tablets to provide appropriate doses to children. Int J Pharm. 2018;545(1-2):57-63. doi:10.1016/j.ijpharm.2018.04.054

